Paracetamol (acetaminophen) is generally considered to be a weak inhibitor of the synthesis of prostaglandins (PGs). However, the in vivo effects of paracetamol are similar to those of the selective cyclooxygenase-2 (COX-2) inhibitors. Paracetamol also decreases PG concentrations in vivo, but, unlike the selective COX-2 inhibitors, paracetamol does not suppress the inflammation of rheumatoid arthritis.
It does, however, decrease swelling after oral surgery in humans and suppresses inflammation in rats and mice. Paracetamol is a weak inhibitor of PG synthesis of COX-1 and COX-2 in broken cell systems, but, by contrast, therapeutic concentrations of paracetamol inhibit PG synthesis in intact cells in vitro when the levels of the substrate arachidonic acid are low (less than about 5 mumol/L).
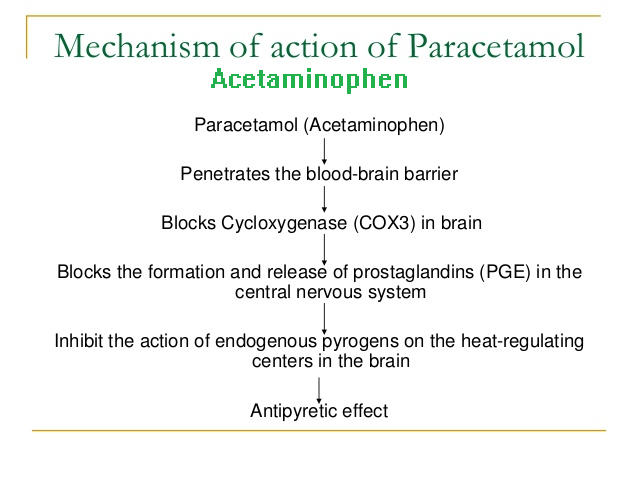
When the levels of arachidonic acid are low, PGs are synthesized largely by COX-2 in cells that contain both COX-1 and COX-2. Thus, the apparent selectivity of paracetamol may be due to inhibition of COX-2-dependent pathways that are proceeding at low rates.
This hypothesis is consistent with the similar pharmacological effects of paracetamol and the selective COX-2 inhibitors. COX-3, a splice variant of COX-1, has been suggested to be the site of action of paracetamol, but genomic and kinetic analysis indicates that this selective interaction is unlikely to be clinically relevant.
There is considerable evidence that the analgesic effect of paracetamol is central and is due to activation of descending serotonergic pathways, but its primary site of action may still be inhibition of PG synthesis.
The action of paracetamol at a molecular level is unclear but could be related to the production of reactive metabolites by the peroxidase function of COX-2, which could deplete glutathione, a cofactor of enzymes such as PGE synthase.